 |
| Chlorine atom |
 |
| Beryllium atom |
 |
| Neon atom |
Here is some of the basic information for each of the elements
Neon- Atomic mass 20.1797 Atomic Number 10
Chlorine- Atomic number 17 Atomic Mass 35.453
Beryllium- Atomic number 4 Atomic Mass 9.012182
For my models, the electrons and the protons equal each other out
Chlorine- 17 protons and electrons
Neon- 10 protons and electrons
Beryllium- 4 protons and electrons
Exciting electrons- When an electron is "excited" they move to a higher energy orbit and when they "relax" they emit photons (light). Now, this light can be in different colors. This is because every element has different numbers of electrons and then different energy levels. Color will change based on the energy levels.
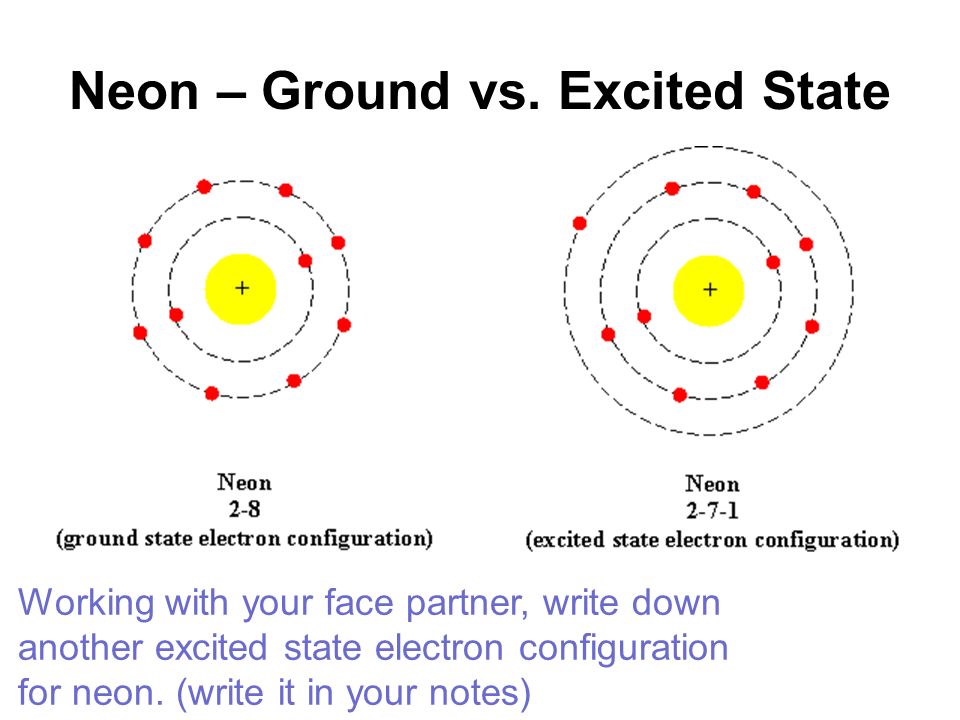 |
| Here is an example of what happens to Neon when it is exciting |
New Year's Eve Fireworks
Fireworks are made up of different elements and when heated and using incandescence. For example, heating oxygen turns it into a blue color.
 |
| Here are some examples of different elements having colors from explosions |
Isotopes
An isotope is a combination of an element but the number of neutrons is different. For beryllium adding three neutrons makes it 7BE.
Fun fact! 99% of an atom is empty space which makes up the majority of the volume in an atom. The remaining 1% is the protons, neutrons, and electrons
The periodic table- Elements in the periodic table are arranged by increasing mass, atomic number, and elements in columns are grouped together to be called groups
Examples of groups and classes
alkali metals- lithium and sodium
alkaline earth- calcium and bromine
Nobel gases- neon and krypton
Transition metals- iron and gold
non-metals- oxygen and sulfur
metalloids- boron and silicon
Crazy Bizarre electrons
After watching Doctor Quantum on behaviors of electrons I learned that electrons can emit both waves and particles. Looking at the double slit experiment, depending on what is present electrons can change behaviors because the environment has changed. But the electron is not changing behavior because there is an observer, there are changes because the environment was affected. The most common example is a fridge light. When the fridge is closed the light is off. When the fridge is open the fridge is on. When a person opens the fridge the light has to turn on. We can't expect the light to stay off even though we opened the door.
No comments:
Post a Comment