2. At the pH Scale PhET simulation, complete the For Teachers activities “pH Scale inquiry-based intro to acid-base” posted by Trish Loeblein on the pH Scale simulation at PhET (http://phet.colorado.edu/en/simulation/ph-scale). On your blog post the answers with your scientific explanations from the “Clicker_questions_pH_Scale.pdf ” posted by Trish. NOTE: As you work with PHET, you may need to create an account at this site.
For question one on the clicker questions it is incorrect. The color of a substance does not indicate if it is an acid, base or salt. The litmus test indicates weather a substance is one of the three. If the litmus test turns red then it is an acid. If it turns blue it is a base.
For question 2 it asks using an image, which of the solutions is basic. Using the pH scale we know that for a solution to be a base it needs to have a ph between 7 and 14. Sample B has a pH of 7.4 and sample C has a pH of 12.06 making two out of the three samples bases.
Question 3 has drawing of H3O and OH on a molecular level. Out of the three options one of them is acidic. The answer is the third image because of the number of hydrogen molecules. That’s what the H in PH stands for. Image 1 is comprised mostly of oxygen molecules. Image b has an equal number of hydrogen and oxygen molecules. Hat levels image C with the largest number of hydrogen molecules. These are also called hydronium Ions.
Question 4 is asking which solution is basic based on the amount of substances in each section. By observation, sample A is neutral with equal parts in the solution. Sample B has more oxygen ions in it. Sample C has more hydrogen ions in it. The correct answer is sample B.
Question 5 is asking which solution is acidic based on the makeup of the three samples. The answer is that samples A and B contain more hydrogen ions and if neutralized will still be acidic.
Question 6 is asking about how adding water will affect the pH of a solution. Water is the most neutral substance so adding it to the solution that has a pH of 5 will make it it be less acidic and increase the pH.
Question 7 asks the reverse question, what happens to the substance if the pH has water added to it. Again, because water is neutral the pH will decrease and lesson the substance ability to be a base.
Question 8 has three susbtances a) pH 6.5 b) pH 7.40 and c) pH12.06 and asks to order them from most acidic to most basic. Looking at a pH scale, 7 is the most neutral substance. 14 is the strongest a base can be and 0 is the strongest an acid can be. The order is A,B,C.
Question 9 shows a bar graph and asks the same question. Order the substances from most acidic to most basic. Option A has a neutral pH of 7, C has a more acidic pH and B has a more basic pH. The order is C,B,A.
Question 10 “ If spit has a pH of 7.4 what does that tell you about the water equilibrium?”. The pH is not at 7 which means something was added to make the solution more basic and it had an effect on the oxygen.
3. At the Acid-Base Solutions PhET simulation, complete the For Teachers activities “Strong and Weak Acids” posted by Kelly Lancaster, Laurie Langdon on the Acid-Base Solutions simulation (http://phet.colorado.edu/en/simulation/acid-base-solutions) and post on your blog your data and answers to the questions posed in their exercise, "Acid_CU_GenChem2_Activity ".
4. Also post on your blog the common name, chemical name and chemical structure for a common acid and base that you commonly use
An acid found in my home is Citric acid. (H3C6H5O7) IUPAC name is 2-Hydroxypropane-1,2,3-tricarboxylic acid. It has a pH of 2.2
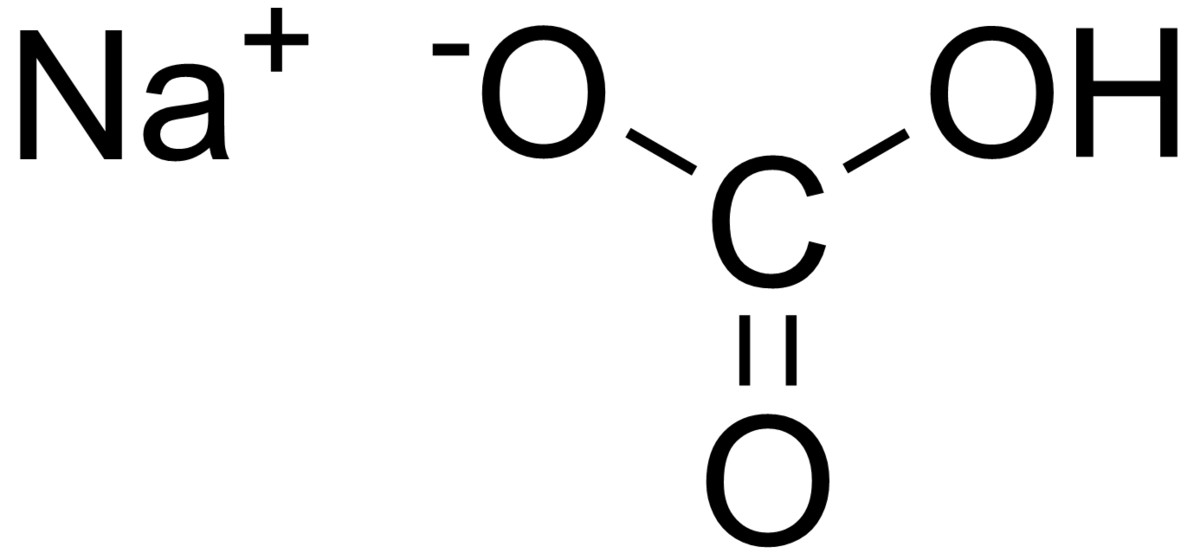
A common base found in my home is Baking Soda, Sodium Bicarbonate is the chemical name and it has a pH of 8.3
No comments:
Post a Comment