Because I'm studying to be an early childhood education teacher I feel I have a more imaginative mind when it comes to science. I chose these because I felt they looked like something other than ball and stick models. |
| chloroform (Trichloromethane) CHCL3 |
 |
| Ammonia (azane) NH3 |
 |
| methanol CH3OH |
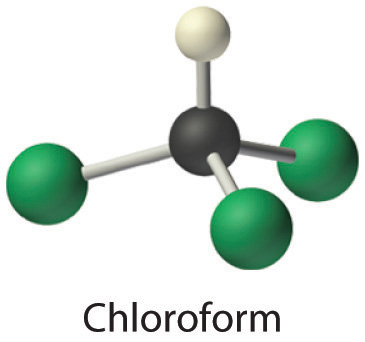 |
| chloroform ball and stick model |
 |
| ammonia ball and stick model |
| ball and stick of methyl alcohol |
IUPAC stands for International Union of Pure Applied Chemistry
Common Chemicals that are so much more
We have so many everyday products that are much more complex than we realize
Baking Powder- NaHCO3; sodium bicarbonate

toothpaste- Sodium Fluoride mixture with triclosan C12H7Cl3FNaO2

salt- NaCl; Sodium Chloride

vinegar- C2H4O2 acetic acid

over the counter aspirin- C9H8O4; acetylsalicylic acid

soap- C₁₇H₃₅COONa sodium stearate

borax- sodium tetraborate decahydrate Na2B4O7.10 H2O

Epsom salt- magnesium sulfate heptahydrate MgSO4.7 H2O

rubbing alcohol- isopropyl alcohol (CH3)2CHOH

cream of Tartar- potassium hydrogen tartrate KHC4H4O6

Tums- calcium carbonateCaCO3

Drano- sodium hydroxide NaOH

Graphite- Carbon C

Glue- polyvinyl acetate C4H6O2

Alcohol- C2H60 Ethanol
Baking soda- Sodium Bicarbonate NaHCO3

Water- Dihydrogen Monoxide H2O

Chocolate- Theobromine C7H8N4O2

Vaseline- Petroleum C15H15N
Caffeine- 1,3,7-Trimethylpurine-2,6-dione C8H11CIN4O2

Carbon has about 4 bonds
Hydrogen has 1 bond
Oxygen has two bonds
5. As you explore ingredients, notice how everything around us is made up of chemicals consisting of atoms bound together into molecules. But what about companies that claim their products are chemical free! How can this be? Here is an example: http://www.naturalhealthcareproducts.com/Cleaning-Products.php
Do a little web searching and propose what chemicals are actually in this product. Keep in mind, that everything at the molecular level is a chemical, whether it be made in nature or in a lab.
Looking at the website from what I can find there are no “harmful” chemicals. What they might mean is that all chemicals in the product are naturally occurring and not artificially created. For example, the laundry detergent on the site contains eucalyptus oil instead of chemicals that create an artificial scent. It allows a person to have peace of mind that toxic chemicals are not present in their products that will harm them over time.
6. Also do a little searching on the web and share on your blog how many chemicals are typically found in things like coffee, milk, beer or whiskey? Only need to choose one.
Chemicals found in coffee- caffeine, water, ethylphenol, quinic acid, dicaffeoylquinic acid, dimethyl disulfide, acetylmethylcarbinol, putrescine, trigonelline, niacin, theophylline
No comments:
Post a Comment